By Suradip Das
Global crisis has always produced groundbreaking innovations
The Spanish Flu that started during 1918, just after WW1, triggered widespread research into viruses, especially Influenza viruses and subsequently led to the Influenza vaccine. Most of the modern technologies that we take for granted today are a by-product of WW2. Penicillin, the broad spectrum antibiotic, being one of the most prominent.
The present pandemic is no different. COVID-19 has upended lives and livelihood across the globe. Due to the severe economic fallout, companies are crumbling, and industrial sectors are hit hard leading to drastic rise in unemployment. As large conglomerates are struggling to survive, small start-ups are coming up every day with innovative solutions leveraging cutting edge science and technology aimed at mitigating some of the issues. India has been no exception. Indian startups have always fostered the spirit of Frugal (or “Jugad”) Innovations, which is a combination of HIGH science and CHEAP technology. The current pandemic has seen hundreds of proposals from indigenous startup companies within the last few weeks aimed at providing cost-effective solutions towards developing diagnostics, therapeutics and healthcare infrastructure to combat the overall burden of COVID-19. Many of these startups are being funded through various schemes of the government and can be traced back to colleges and universities where entrepreneurial students have joined forces with expert faculty members. In addition to startups, many indigenous private biomedical companies have completely repurposed their production pipeline almost overnight to start producing testing kits, protective wears and ventilators.
This article is about providing a snapshot of some of the most exciting examples of Frugal Innovations in diagnostics and therapeutics that India has come up with to combat this pandemic.
INNOVATIONS IN DIAGNOSTICS
MyLab Discovery Solutions – This is one of the most promising startup companies that have been in the news recently for their rapid testing kit. PathoDetect CVD 19 Qualitative PCR kit developed by this Pune based startup is the first indigenous testing kit that has been approved by ICMR. The kit reduces the testing time from 7-8 hours to 2.5 hours, drastically reduces the cost (from usual Rs 4500 to Rs 1200/kit) as well as increases throughput to 25-100 reactions per kit.

Steps in the Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) test: a) Specimen is taken from the nose or throat of individual b) RNA is extracted and c) is transcribed into complementary DNA (cDNA) , d) Once the primers have bound to the DNA, they provide a starting point for the DNA polymerase to help copy it. DNA polymerase then degrades the bound probe which results in an increased fluorescence signal e) The fluorescence increases as copies of the virus DNA are made. If the fluorescence level crosses certain threshold, the test is positive. Source – Globalbiotechinsights.com. Copyright 2020 – IDTechEx
This is achieved by innovations across various stages of the testing process – starting from using magnetic bead technology and a proprietary enzyme (Proteinase K) solution that significantly enhances the efficiency of viral nucleic acid extraction to reducing the required concentration of expensive enzymes like Reverse Transcriptase (converts RNA to DNA) and Taq polymerase (amplifies the target viral DNA) by 50% using a unique buffer solution thereby significantly reducing the cost of each reaction. With recent investments by Serum Institute Bangalore, MyLab is expected to ramp up production to enable 20 lakh tests per week.
This startup is being propelled by a strong team comprising of postgraduate students, experienced virologists with experience in working with leading biotech companies as well as regulatory specialists, supported by people from strong finance, sales and marketing background. Mrs. Minal Dakhave Bhosale, head of R&D at MyLab, who led the team working day and night amidst her own complicated pregnancy and developed the kit in 6 weeks, remains an inspiration for all scientists who are working in labs across the globe trying to fight this pandemic.
Molbio Diagnostics/Bigtec Labs – Molbio Diagnostics is another privately owned diagnostic company with an array of molecular biology products. Their claim to fame is through the development of a portable, battery operated PCR machine that has been approved for TB testing by ICMR and WHO. Bigtec Labs, which is the R&D wing of Molbio Diagnostics, has managed to use the same platform to come up with Truenat™ Beta CoV, which is a chip based realtime PCR kit approved by ICMR for screening of COVID-19 cases.
The central innovation lies in the microPCR technology that enables point-of-care diagnostic capabilities in a cost effective manner (Rs 1350 approx/test). Additionally, the results can be obtained within an hour and transmitted wirelessly making the system ideal for rapid screening of population in semi-urban and rural areas.
Huwel Lifesciences – Most of the present day diagnostic kits for COVID-19 are PCR based. Polymerase chain reaction or PCR requires multiple enzymes for viral nucleic acid extraction and amplification of target sequences. It is noteworthy, that many of these enzymes are supplied by China. Keeping in mind the current supply chain and logistical challenges of healthcare equipment especially from China, it is crucial to develop indigenous manufacturing capabilities.
Huwel Lifesciences, a Hyderabad based company, attempts to precisely mitigate this scenario by developing a diagnostic kit, such that every component of the kit including the machine, sample transportation medium and enzymes, are made in-house. The company formed in 2015 by Dr. Shesheer Kumar and Dr. Rachana Tripathi has a GMP compliant manufacturing facility that enables them to achieve such complete independence in terms of manufacturing. Their Quantiplus® CORONA VIRUS (2019nCoV) Detection Kit was recently approved by ICMR for COVID screening. The company is currently producing 3,000-4,000 kits per day.
NuLife Care – Although PCR remains the gold standard in terms of detecting SARS-COV-2 viral genetic material, it is a lengthy process taking upto 7-8 hours other than being non-trivial requiring trained personnel. Our body produces molecules called antibodies to fight against infections like coronaviruses. Antibody tests presents an alternate indirect way of screening a population by looking for antibodies against coronavirus in the blood. Antibody tests are usually very fast (around 15 min) and can be performed by semi-skilled personnel.
A health worker performing an antibody test for Covid-19 at a hospital in Poland. Photograph: Omar Marques/Getty Images Source – The Guardian
Noida based Nulife Care has come up with a rapid Antibody Test (Abchek COVID-19 IgM/IgG) that can use blood from a finger prick and give results within 15 mins. Dr. Nadeem Rahman, an alumnus of Aligarh Muslim University along with his team was able to develop the kit within a few weeks with logistical support from the Uttar Pradesh government that enabled smooth functioning of the lab even during the lockdown. The kit which costs around Rs.500-600 have been approved by ICMR and the company has a production capacity of 1lakh kits per day.
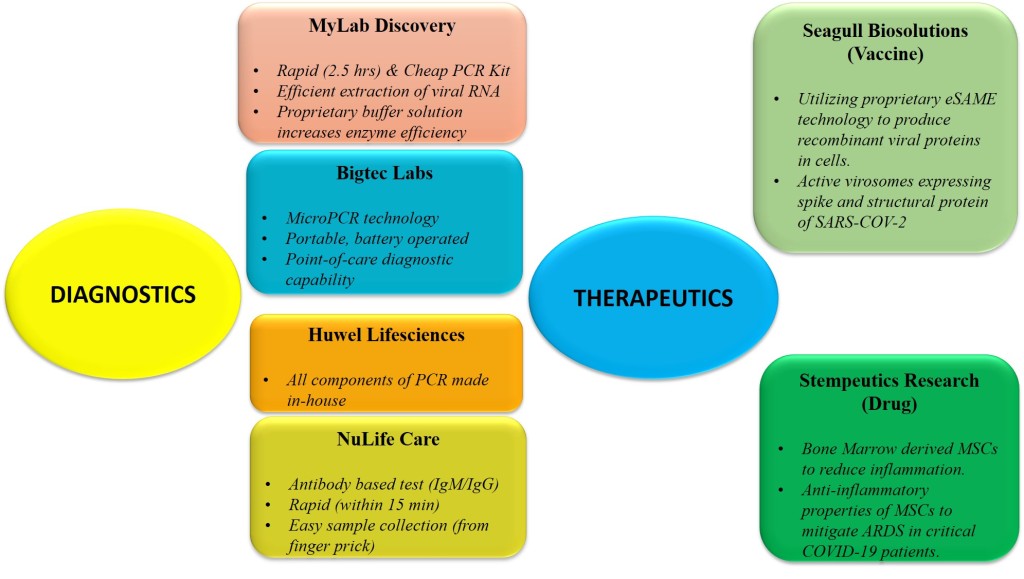
Examples of Indian Innovation towards developing diagnostics and therapeutics for COVID-19. Copyright 2020 Getinsight.blog
INNOVATIONS IN THERAPEUTICS
Seagull BioSolutions – This Pune based startup is one of the few Indian companies working on developing a vaccine against SARS-COV-2. Seagull Biosolutions was founded in 2011 by Dr Vishwas Joshi (PhD in molecular virology) along with Dr Shailendra Maheshwari (MD in radiology) and was incubated at Venture Center, an Entrepreneurship Development Center, hosted by National Chemical Laboratory (NCL), Pune.
Technologies developed by Seagull Biosolutions is based on two central innovations –
(1) eSAME platform – Used to express recombinant proteins and RNA products in different animal cells.
(2) Active Virosome Technology – Virosomes are spherical structures comprising of a phospholipid membrane and a cavity. The membrane has viral glycoprotein molecules which help the virosome to fuse and enter host cells whereas the cavity contains genetic components of a “killed” virus. They aim to utilize this technology to develop two types of Active Virosome agents – one expressing S protein of COVID-19 (AV-S) and another one expressing structural proteins of COVID-19 (AV-SPs). Once developed, these will be tested in mice to evaluate their ability to induce anti-COVID-19 neutralizing antibodies & cellular immune response(s).
The company has won several awards and research funding from DBT, DST and Bill and Melinda Gates Foundation. Their current COVID-19 vaccine technology is being supported by DST, Govt of India. In terms of the expected timeline for COVID vaccine, Seagull Biosolutions “aims to complete the proof of concept in 80 days & complete preclinical development & start Phase I trial by the end of 18-20 months.”
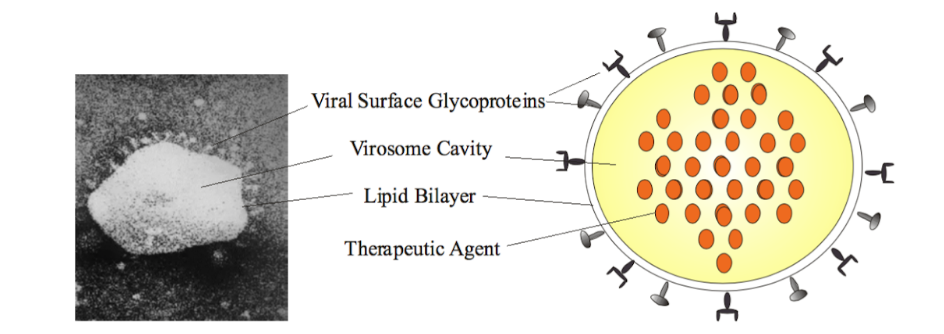
Components of a Virosome. Source: Wikipedia/ Babar et al. / CC BY-SA (https://creativecommons.org/licenses/by-sa/4.0)
Stempeutics Research – This Bangalore based company, which is part of the multi-billion dollar Manipal Education and Medical Group, is arguably the leader in stem cell research in India. Stempeucell, their Mesenchymal Stem Cell (MSCs) derived drug, is presently in advanced stages of clinical trial in India for treating Critical Limb Ischemia and Osteoarthritis by reducing inflammation. Based on their experience of using bone marrow derived MSCs for its anti-inflammatory properties, Stempeutics Research has applied for permission to test their drug on critical COVID-19 patients suffering from Acute Respiratory Distress Syndrome or ARDS. Although their MSC based products have been found to be safe so far, their efficacy in reducing inflammation and mitigating ARDS in COVID-19 patients is yet to be proven.
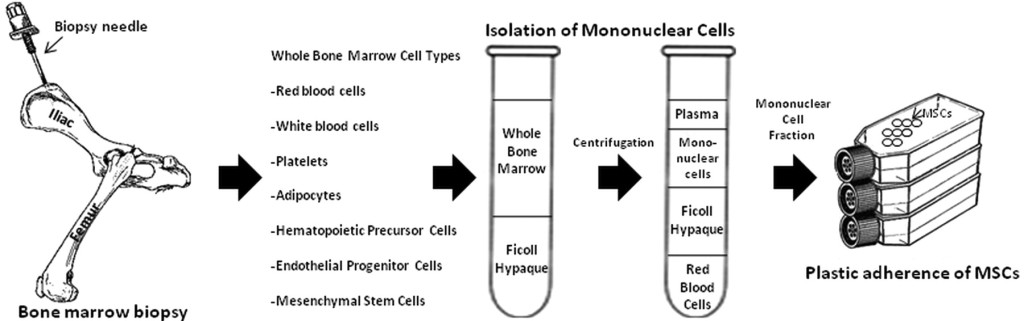
Isolation of mesenchymal stem cells (MSCs) from a bone marrow biopsy. Many stem cells and maturing cells exist in the bone marrow cavity. The mononuclear cells are isolated from red blood cells by Ficoll density centrifugation, and the MSCs are separated from the mononuclear cells by plastic adherence in culture. Source – Williams et al, Circulation Research. 2011;109:923–940.
The above examples are in no way exhaustive and is only aimed at providing an overview of innovations arising out of Indian Biotech companies during this pandemic. As we stay indoors and work from home, we are indebted to our first responders and healthcare professionals.
In this spirit, let us also take a moment to express our gratitude to the thousands of scientists in India and worldwide who are working around the clock behind the scenes in laboratories to combat this crisis. They remain our last and only hope for returning to normalcy.
Read our article on COVID-19 Clinical Trials in India to get a glimpse of the wide variety of products undergoing clinical trials to evaluate efficacy against the coronavirus.
Stay tuned for more articles on COVID-19 as I attempt to declutter the scientific advances in the field.
P.S. For a more comprehensive information about various innovations and funding opportunities regarding COVID-19 in India, please visit the following links –
(1) A list of innovations currently supported by BIRAC, DBT, Govt of India. https://birac.nic.in/webcontent/1585918972_covid_solution_v2.pdf
(2) Existing COVID related funding opportunities for Indian Startup companies. https://www.startupsvscovid.com/
Copyright 2020 Getinsight.blog.


Very informative article. In a concise, yet very comprehensive manner u did explained it.
Thank you Depriyo. I am glad you liked it.
A very necessary one although I couldn’t follow so much.
Thanks for your comment. I tried to tone down the scientific jargon as much as possible. Please let me know if there are specific issues that you might want me to clarify. I am still learning to blog and will keep trying to write in more simple language in future.
Fight against COVID19
To fight against COVID19 India has to chalk out her own plan; we should not ape other nations. There would be serious socio-economic disaster in India if we continue total or partial lockdown. Most of the COVID19 infected patients in India are asymptomatic; and this might be due to presence of Curcumin in our daily food. So, it is very difficult to determine patient- load in India; we can not test for COVID19 in all persons under the Sun.
Asymptomatic persons would refuse testing and any form of treatment – they would roam freely in society; and would infect the population at large; and this will in near future usher in HERD IMMUNITY in India. Most of the symptomatic patients present with mild to moderate illness; we should treat them as OPD patients. We have to treat these patients with mild-to-moderate Flu-like symptoms as quickly as possible; so that, they do not become severely ill to overwhelm the scarce indoor facilities in our country. Here is a safe outdoor treatment plan that will show quick and effective response in symptomatic patients (for adults): – Vitamin D & C and Zinc daily in usual recommend doses + Virostatic drug Ivermectin 12mg daily for 3 to 5 days + Senolytic antibiotic Azithromycin 250mg to 500mg daily for 5 days to 10 days. This treatment plan ushers in prompt response. Serious indoor patients would get these medicines + necessary supportive measures + Hydroxychloroquine (HCQS). – Arun Kumar Laha 29, Abinash Banerjee Lane. Howrah 711104. (laha.a53@gmail.com)
Disclaimer: –
This is not a prescription for general public to self- medicate themselves. It is for medical fraternity to think about my humble suggestion.
To solve the problems of COVID 19, lockdown is not the only way out; we have to rely on development of HERD IMMUNITY by exposing people to low grade infection with COVID19 and we have to treat the symptomatic patients as fast as possible, to cut short the number of serious patients requiring indoor treatment.
Many health- care personnel and other fighters against COVID19 are losing their lives to save the humanity at large; ICMR should think about prescribing IVERMECTIN (12mg in empty stomach once in a week instead of Hydroxychloroquine) to these persons. Ivermectin is safer than Hydroxychloroquine.
Sir, thank you for sharing your thoughts. I will be writing about some of the possible treatment modalities you suggested in my next blog which will be published soon. Please follow/subscribe to our page so that you get an instant notification. I look forward to your comments on my next blog as well.
May be you can mention the kit developed by IIT Delhi and marketed by GeneI. Much reduced price and equivalently reliable.
Thanks for your comment. As I mentioned, it’s just an attempt to showcase some examples. When the post was published, GeneI hadn’t yet partnered with IIT Delhi. Hence I had omitted it. I was trying to restrict myself to examples which were already commercially available. Thanks again for reading and sharing your thoughts. Please follow/subscribe our blog. The next article is coming soon and we look forward to hearing from you.