The recent COVID-19 pandemic is speculated to have originated in Bats which transmitted the virus into intermediary hosts like Pangolins. These intermediary hosts eventually transmitted the virus into humans. Since then, Bats have received a lot of “negative press” as the harbinger of the Devil. Ecologists have constantly tried to educate how important Bats are for maintaining our ecological balance and are indeed a cornerstone for our environment.
Bats as reservoir of viruses
Bats act as a natural reservoir for a variety of viruses ranging from Ebola and Rabies to a whole gamut of coronaviruses like SARs and MERs. Several genome sequences of bat coronaviruses have been reported that show a high genetic similarity to SARS-CoV-2. It should also be emphasized that bat-borne viruses cause devastating outbreaks not only in humans, but also in animals such as pigs and horses. For example, the SARS-COV-2 virus has been shown to be capable of anthropo-zoonotic (from humans to other animals) transmission to minks, cats, dogs and even tigers and lions in zoos. Indeed – bats host more zoonotic pathogens than any other known mammalian species.
The question thus arises – Why do not bats get infected with all these viruses?
How Bats use flight to fight viruses
In general, there are two hypotheses behind bats ability to fight/tolerate viruses, although not mutually contradictory. One school of thought attributes the ability of bats to fight viruses to its flight capabilities. Interestingly, bats are the only flying mammals. Flight is metabolically expensive in that it requires a lot of energy. During flight, bats consume 1200 calories per hour, metabolic rate increases 2.5-3 times and heart rate can go up to a 1000 beats per minute. Further, in flight body temperature increases to 410C. Scientists postulate that this daily cycle of high metabolic activity accompanied by “fever” forestalls viral replication and activates the host immune system. On the other hand, viruses which survive such ecological pressure within the bat are more resistant to febrile responses while infecting humans or other animals.
The other school of thought argues that the unique immune system of Bats, as detailed in the following sections, allows it to tolerate large viral loads while remaining asymptomatic.
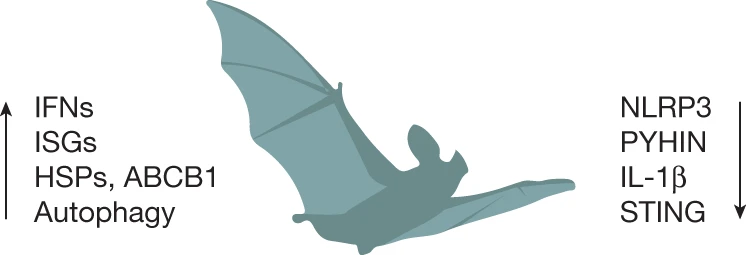
Enhanced host defense in Bats against viruses
Bats, like humans, produce a range of molecules called interferons (IFNs) in response to viral infection. These interferons along with other interferon stimulated genes (ISGs) help to reduce viral replication. Compared to humans, Bats produce much higher amounts of these molecules that help them fight viruses more efficiently. Further, Bats cells exhibit higher autophagy – a process by which cells flush out viral particles. Bats also express very high levels of Heat Shock Proteins (HSPs) – which allows Bat cells to survive higher temperatures (during flight) and tolerate viral mutations.
Dampened inflammatory reaction helps Bats tolerate viruses
The immune system is like our internal security forces made up of cells and proteins that constantly keep vigil to detect any external pathogen and immediately respond to infection. A group of proteins called Inflammasomes, located inside our cells act as sensors for detecting pathogens and raises an alarm through secretion of different chemicals (like interleukins) initiating a process of “fight back” called inflammation. This process of body’s natural tendency to fight back external pathogens gives rise severe symptoms like fever, tissue damage and overall systemic shock. It is actually our body’s inflammatory response to SARS-COV-2 coronavirus, rather than the virus itself, that leads to severe symptoms and fatality. Some of the most studied components of the Inflammasome are the proteins – NLRP-3 and STING, that senses microbial/viral motifs, endogenous danger signals and environmental irritants. NLRP3 is increasingly recognized for its role in response to multiple viruses, including those associated with bats, such as influenza A virus and rabies virus. Viral infection in humans leads to hyperactivation of NLRP-3 inflammasomes sending our immune system in overdrive which ultimately leads to pathological symptoms.
While human immune system tends to go out of control upon viral infection (especially bat borne viruses), bats have found a way to dampen/switch off their immune response in the presence of viruses. Specifically, scientists have found that Bats tend to reduce production of NLRP-3 protein as well as decrease their functional capacity. This allows Bats to remain asymptomatic even when the virus continues to replicate within them.
Bat inspired potential novel therapeutics
If Bats can survive viral infections by reducing NLRP-3 protein, can we not have medicines that can do the same? The answer is Yes, we can, and this has already been done in case of other diseases.
NLRP-3 is suspected to be involved in pathological symptoms related to a wide range of diseases like Alzheimer’s disease, atherosclerosis, inflammatory bowel disease and rheumatoid arthritis. Excitingly, a number of NLRP3 inhibitors have been synthesized to date, that can either directly inhibit NLRP3 or indirectly attenuate related signaling events. One of the most potent inhibitors of the NLRP-3 protein that has been designed so far is a chemical called MCC950. MCC950 has been tested in 80 animal models of more than 50 diseases and has shown promising results in a range of diseases like experimental autoimmune encephalomyelitis, Alzheimer disease, traumatic brain injury etc. A major point of concern regarding such inhibitors is the present lack of knowledge about their specific mechanism of actions and potential side effects.
Can we use this knowledge about NLRP-3 inhibitors to treat viral infections especially COVID-19?
During this current pandemic, the virus SARS-COV-2 has mutated multiple times, often presenting with higher infection rate and severity (Link to article on COVID mutations). Hence, in addition to vaccines, we must develop therapeutic strategies that target the inflammatory pathways which lead to severe symptoms. This is because, regardless of mutations, the mechanism of action of the virus mostly remains the same.
Respiratory failure following SARS-COV-2 infection is the result of dysregulated hyperinflammation. This pathology is characterized by hyperactivation of NLRP3 inflammasome pathway and release of its products including the proinflammatory chemicals. In a recently concluded phase II clinical trial by a UK group, it has been found that a commonly used drug among asthma patients called Budesonide reduces the severity of COVID-19 symptoms. Interestingly, Budesonide is an inhaled steroid that attenuates the NLRP-3 inflammasome pathway and thereby prevents lung damage when administered at an early stage of the disease.
The physiology of Bat and its ability to remain asymptomatic after viral infection has long been an interest to scientists and this field of research has got a special impetus after the COVID-19 pandemic. We can expect many more drugs like Budesonide to be studied and made available for therapeutic use taking inspiration from Bat’s ability to fight viruses.
References and Further Reading
- Lessons from the host defenses of bats, a unique viral reservoir – Click here
2. Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host – Click here
3. The NLRP3 inflammasome – Click here
4. Could an NLRP3 inhibitor be the one drug to conquer common diseases? – Click here
5. Inhaled budesonide in the treatment of early COVID-19 – Click here